Joshua Lederberg, a renowned geneticist and microbiologist, once highlighted the formidable challenge viruses pose to humanity by saying, “The single biggest threat to man’s continued dominance on the planet is the virus.”
This stark perspective is especially relevant to the discussion of U.S. Patent 10130701, which addresses the complex world of virology and vaccine development against the Infectious Bronchitis Virus (IBV), a coronavirus affecting poultry. This patent introduces a groundbreaking genetic modification to reduce the virus’s pathogenicity.
While the patent was initially misinterpreted as related to human coronaviruses, the patent’s true focus and innovation lie in advancing poultry health.
This article seeks to explore the nuances of this patent, underlining its crucial role in veterinary vaccine development and its broader implications in the realm of virology.
A Brief Overview of the U.S. Patent 10130701
U.S. Patent 10130701 pertains to a mutated form of avian infectious bronchitis virus (IBV), a coronavirus that affects poultry but not humans. This patent was issued on November 20, 2018, to the Pirbright Institute, a UK-based research organization specializing in preventing viral diseases in livestock. The mutations introduced in the virus were designed to weaken it, allowing it to be used as a vaccine to protect chickens from IBV. The patent does not relate to the novel coronavirus (2019-nCoV) that affects humans.
Source- Science Direct
The patent outlines a method for creating such a virus, which includes transfecting a plasmid into a host cell, followed by infection with a recombining virus containing a coronavirus strain’s genome. The process allows for homologous recombination between the replicase gene sequences in the plasmid and the corresponding sequences in the recombining virus genome. This produces a modified replicase gene, leading to the development of a recombinant coronavirus with the altered replicase gene.
It’s important to note that the Pirbright Institute focuses on research on animal coronaviruses, primarily chickens, and does not work with human coronaviruses. The institute’s patented work on the avian coronavirus was not funded by the Gates Foundation, though the foundation does provide funding for the institute for other projects. The Gates Foundation is actively involved in combating diseases and has committed funds to help contain the spread of new coronaviruses, but this is unrelated to the patent in question.
Historical Context Of The U.S. Patent 10130701
U.S. Patent 10130701 is set against a backdrop of rapid advancements in poultry health research. This patent, outlining a novel form of coronavirus with a genetically modified replicase gene for reduced pathogenicity, is part of a larger effort to combat infectious diseases in animals.
Recent research in the field has explored various innovative strategies for improving poultry health and food safety. For instance, studies have focused on using natural compounds, bacteriophages, and nanoparticles to enhance immune responses in poultry and combat bacterial resistance, which is crucial for ensuring food safety and extending the shelf life of poultry products.
These efforts are essential in a global context where diseases like infectious bronchitis, targeted by the patent, pose significant economic and public health challenges. The patent’s focus on vaccine development for infectious bronchitis in poultry aligns with these broader research goals, highlighting its relevance in ongoing efforts to improve poultry health.
The Inventors Behind U.S. Patent 10130701
The inventors behind US Patent 1030701, Erica Bickerton, Sarah Keep, and Paul Britton, have made significant contributions to the field of virology, particularly in the study of coronaviruses.
Erica Bickerton, a molecular virology expert, stepped into the limelight in 2016 as an Institute Fellow and soon after, in 2018, took the helm of the Coronavirus group. Her battlefield: the infectious bronchitis virus (IBV), a formidable foe in the world of poultry farming. Bickerton’s mission has been to decode and tame this virus, a pursuit she’s undertaken alongside major players in veterinary pharmaceuticals and various bioscience research committees.
Paul Britton stands as her ally in innovation, contributing significantly to patents, particularly those crafting novel coronavirus proteins and vaccines aimed at infectious bronchitis. His research focuses on developing vaccines with broader efficacy, targeting diverse virus strains.
Completing this triumvirate is Sarah Keep, whose collaboration with Bickerton and Britton has led to the development of patents for live, attenuated coronaviruses. These specially engineered viruses, altered at key protein sites, are intended as vaccines to prevent diseases like infectious bronchitis.
The trio’s collaborative efforts, especially in developing live, attenuated coronaviruses and mutant spike proteins, represent a major stride in vaccine development for animal health. Their work stands at the forefront of efforts to control and prevent poultry diseases, an essential factor in maintaining global food security and animal welfare.
Current Relevance Of U.S. Patent 10130701
U.S. Patent 10130701, related to a coronavirus used in developing animal vaccines, has specific relevance in the current world health scenario in several ways:
Veterinary Health
The patent focuses on a coronavirus used in vaccine development, primarily targeting infectious diseases in animals, such as infectious bronchitis in birds. In the current global health context, where zoonotic diseases (diseases that can be transmitted from animals to humans) are a significant concern, the relevance of such vaccines becomes even more critical.
For instance, the origin of HIV/AIDS is traced back to simian immunodeficiency viruses (SIVs), demonstrating the zoonotic nature of some of the most impactful human diseases. This highlights the necessity of vigilant monitoring and management of animal health to prevent future zoonotic transmissions.
Research and Development
The technology and methodologies involved in this patent can contribute to continuous research and development in the field of virology and immunology. Understanding the mechanisms of virus attenuation and vaccine development for animals can offer insights that may be applicable in broader contexts, including human health.
Global Pandemic Preparedness
The COVID-19 pandemic has highlighted the importance of rapid vaccine development and understanding coronavirus biology. While this patent is not directly related to SARS-CoV-2, the learnings from any coronavirus research, including those covered in this patent, can potentially inform strategies for dealing with future coronavirus outbreaks or pandemics.
One Health Approach
The ‘One Health‘ concept, actively recognizing the interconnectedness of human, animal, and environmental health, is increasingly coming to the forefront. The patent aligns with this broader context as this approach extends beyond direct disease control in animals.
It encompasses a holistic view, acknowledging that enhancing animal health, as facilitated by this patent, indirectly contributes to the overall environmental health. This comprehensive perspective underlines the importance of integrated strategies in disease management and health promotion across all domains.
Ethical and Legal Discussions
The patent also contributes to ongoing ethical and legal discussions about patenting life forms and genetic material. These discussions are critical as they shape the policies and regulations that govern scientific research and its application in society.
Controversies And Implications Regarding U.S. Patent 10130701
U.S. Patent 10130701, while distinct from COVID-19, has sparked considerable controversy in the realm of virology. The crux of the debate centers not on its association with human coronaviruses but on the ethical and scientific implications of manipulating a coronavirus, in this case, the avian infectious bronchitis virus (IBV).
Some speculations wrongly suggested the patent as evidence of the artificial creation of viruses, leading to a significant misunderstanding in public discourse. The controversy surrounding this patent underlines a critical aspect of virology research: the ethical considerations of virus manipulation for vaccine development. Patents in this field, like U.S. Patent 10130701, are often aimed at isolating genetic material or inducing mutations in viruses to facilitate research and vaccine production. However, the broader implications of such research, especially in light of public misinterpretations, raise questions about transparency and communication in scientific discoveries.
Transitioning to the legal landscape, a pivotal Supreme Court decision in 2012 reshaped the patenting practices in virology. This ruling clarified that genes and the information they encode cannot be patented merely on the basis of isolation. This decision has had a profound impact on how researchers and companies approach patenting in the field of virology, balancing the need to protect and incentivize innovation against the broader principles of accessibility and ethical research practices.
Public Perceptions And Views Regarding U.S. Patent 10130701
The public perception and views regarding the U.S. Patent 10130701 have been significantly influenced by misinformation and conspiracy theories, especially in the context of the COVID-19 pandemic.
Misinformation and Conspiracy Theories
Source- Dimsum Daily
Many public views on this patent have been shaped by incorrect claims circulating on social media. Some of these claims suggested that the patent was for a COVID-19 vaccine or even that COVID-19 was a manufactured disease. These claims have been debunked by multiple fact-checking organizations. For instance, Reuters conducted a fact-check which clarified that the Pirbright Institute’s patent was for a type of coronavirus found in poultry and pigs, and it had no connection with COVID-19. Such misinformation led to a public perception of the patent that was not aligned with its actual scientific and legal reality.
Public Distrust and Skepticism
The spread of misinformation about this patent has contributed to public distrust and skepticism, particularly toward pharmaceutical companies and research institutions. This is part of a broader trend where misinformation can fuel conspiracy theories and mistrust in scientific authorities. FactCheck.org highlighted that the theories regarding the patent were clearly bogus and that no vaccine for COVID-19 existed when these claims were made. This narrative can contribute to vaccine hesitancy and skepticism towards public health efforts.
Role of Social Media
Source: Buzzfeed News
The role of social media in spreading misinformation about this patent is significant. The ease with which unverified information can be shared and go viral contributes to the rapid spread of false narratives. This phenomenon underscores the challenges faced in managing public perceptions and the importance of critical thinking and fact-checking in the digital age.
Streamlining Patent Programs: How TriangleIP’s TIP Tool™ Makes It Easier?
It’s evident that this patent stands as a hallmark of innovation in the field of veterinary vaccine development, specifically targeting the Infectious Bronchitis Virus (IBV) in poultry. This groundbreaking work underscores the importance of intellectual property in safeguarding and capitalizing on such pioneering ideas.
However, navigating the complexities of capturing innovation enterprise-wide and patenting can be challenging, particularly for those who don’t have a systematic business process for mining patents. This is where a tool like the TIP Tool™ by TriangleIP becomes invaluable.
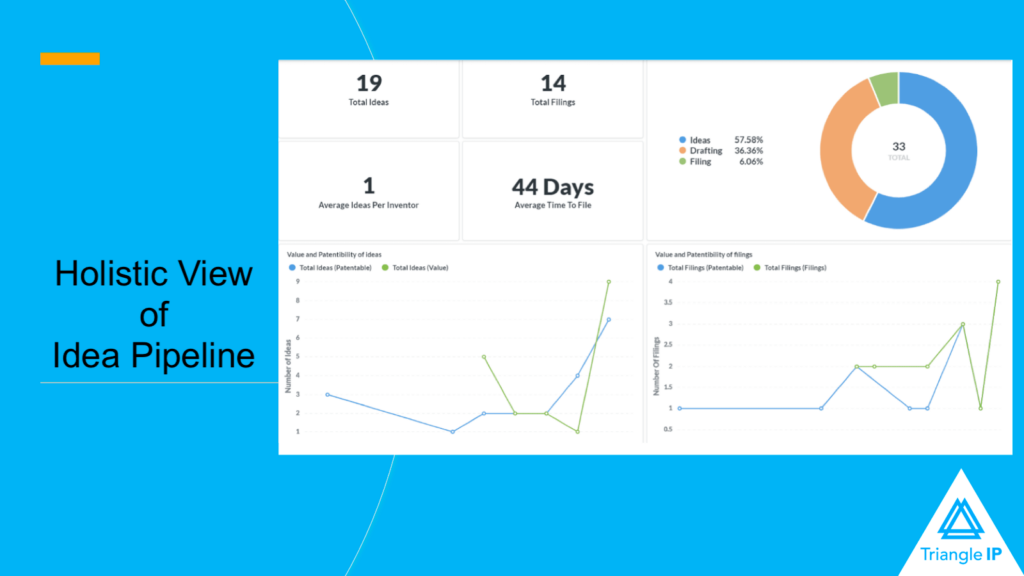
The TIP Tool™ enhances the process of patent mining in various ways:
- It provides a streamlined pathway from the initial idea capture to securing patent protection and strategic patent portfolio management with data-driven insights at each stage.
- Its inventor-friendly idea capture form for collecting ideas and role-based access aids in capturing innovation enterprise-wide without worrying about exposure to sensitive IP information.
- The TIP Tool™ facilitates real-time collaboration amongst innovators, managers, patent counsel, and other members of the patent committee via a Google Doc-like interface within the tool for thorough vetting of ideas.
- The tool also delivers extensive patent analytics, providing insights into patentability, predicted timelines, cost estimates, and up-to-date USPTO status tracking, aiding in informed patenting decisions. Presented below is a glimpse of what the predictions from the TIP ToolTM look like:
| Check Points | The TIP Tool™ Predictions About U.S. Patent 10130701 | Actual Information About U.S. Patent 10130701 |
| Average Allowance Time | 3 years | Applied in 2017, Granted in 2018 |
| Average Argument Rounds | 1.7 | Not Available |
| Average Allowance Rate | 72% | 100% (As it’s an issued patent) |
| End-To-End Parenting Cost Estimation | $40,280 | Not Available |
| Art Unit | 1762, 1731, 1648 | Group Art Unit (GAU) 1648 |
| Examiner Analytics | Li, Bao Q Avg. Allowance Rate – 81%Avg. Argument Rounds – 1.3Avg. Allowance Time – 2 Years | Li, Bao Q |
- The TIP Tool™ offers a comprehensive view of your idea, patent pipeline, and patent portfolio, focusing on ease of management and decision-making.
Streamline your patent program by registering on the TIP Tool™ today!



